Fuster Franck
Maître de Conférences à la Faculté des Sciences et Ingénierie, Sorbonne Université.
Ma Recherche
Mots clés : fonction de localisation électronique, topologie, réactivité, liaison chimique, liaison hydrogène.
Dernières parutions :
- P. Chaquin, F. Fuster, A. Markovits, "How the addition of atomic hydrogen to a multiple bond can be catalyzed by water molecules", Journal of Computational Chemistry, 45(27), 2325-2332, 2024.
- P. Chaquin, F. Fuster, "Quantification du caractère liant/antiliant d’une orbitale moléculaire et visualisation des orbitales atomiques participant à une orbitale moléculaire", Union des professeurs de physique et de chimie, 118, 409-419, 2024.
- Novoa T, Laplaza R, Peccati F, Fuster F, Contreras-García J. "The NCIWEB Server: A Novel Implementation of the Noncovalent Interactions Index for Biomolecular Systems", Journal of chemical information and modeling, 63(15), 4483-4489, 2023.
Thêmatiques :
- Développement de codes scientifiques.
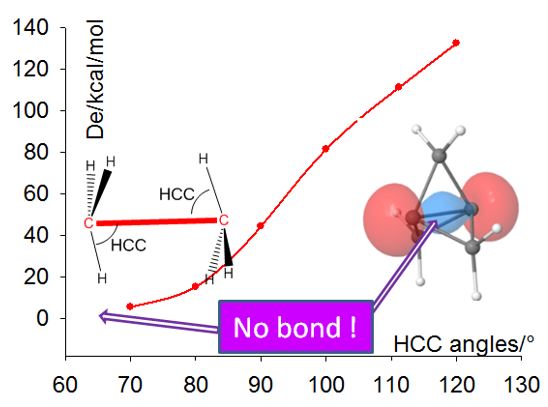
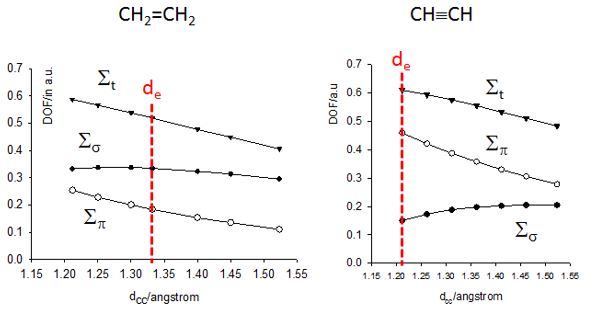
- Estimation du caractère local (anti)liant des OM basée sur les Forces Dynamiques Orbitalaires (DOF).
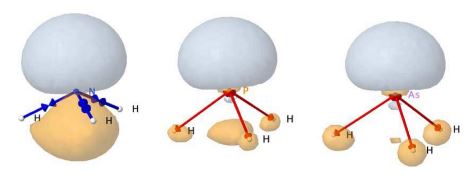
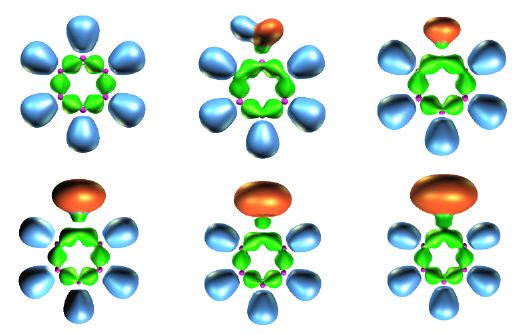
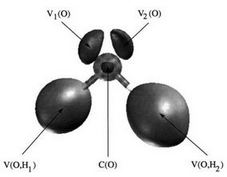
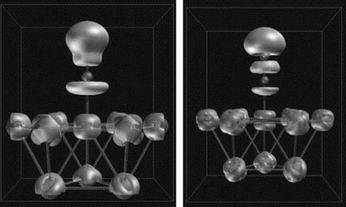
- Méthodes interprétatives : topologie de la fonction ELF.
- Réactivité topologique en chimie organique.
- Caractérisation topologiques des liaisons chimiques dans les molécules organiques et inorganiques.
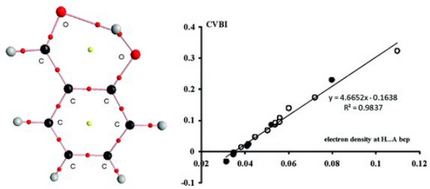
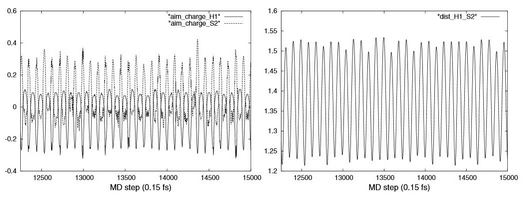
- Descriptions topologiques et interprétations des liaisons faibles : "liaisons hydrogènes" faibles, moyennes et fortes, interactions de Van der Waals.
Méthodologies :
- Calculs ab-initio (DFT) : gaussian16.
- Calculs et interprétations des fonctions locales : densité, laplacien de la densité, ELF, LOL, NCI...
Programmation :
- pyhton 3, tkinter
- fortran77 et 90
- html5, javascript, php
- bash, perl, awk
Codes scientifiques :
- TopMod : suite logiciel de calculs topologiques.
- TopMod GUI version 1.1 (2023) : interface utilisateur pour générer et visualiser les fichiers de TopMod09.
Exemples de la topologie de la function ELF : OrbiMol section topologie (H2O, C2H6, C2H4, C2H2, C6H6, (FH)2, NH3BH3, FHF-, H2O2, H2S2.-, ...).
Cloud computing :
- TopChemWeb v1.0 (2023) est une implémentation Web du code TopChem2.
- NCIweb v1.0 (2022) est une implémentation Web du code NCIplot.