In a linear molecule,
the attractors which are not located on the ![]() axis
are degenerated on circles centered on this axis and lying in planes perpendicular
to the axis direction. The dynamical system of the electron localization
function of a linear molecule is structurally unstable if it possesses
such circular attractors. The convenient graphical representation is a
map in any plane containing the internuclear axis.
axis
are degenerated on circles centered on this axis and lying in planes perpendicular
to the axis direction. The dynamical system of the electron localization
function of a linear molecule is structurally unstable if it possesses
such circular attractors. The convenient graphical representation is a
map in any plane containing the internuclear axis.
There is two types
of attractors: the core attractors and the valence attractors. The core
basins correspond to the core shells of atoms with ![]() ,
they are concentrically organized around each nucleus with
,
they are concentrically organized around each nucleus with ![]() .
The other basins are valence basins.
.
The other basins are valence basins.
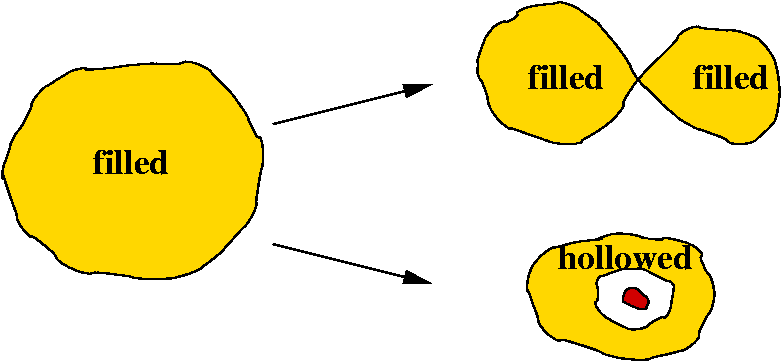
Upon the increase of
the value of ![]() defining the bounding isosurface, a reducible domain split into several
domains each containing less attractors than the parent domain. The reduction
of localization occurs for at turning point which are critical points of
index 1 located on the separatrix of two basins involved in the parent
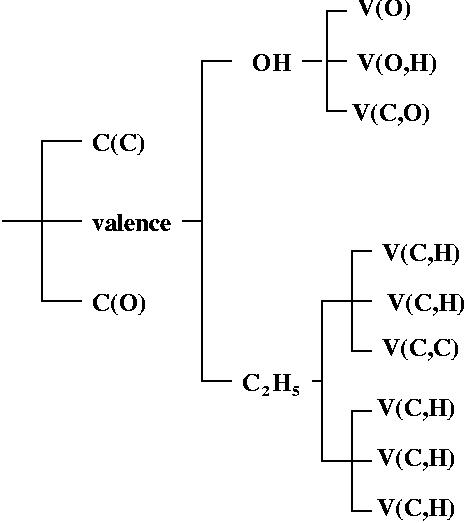
domain. Ordering these turning points (localization nodes) by increasing
defining the bounding isosurface, a reducible domain split into several
domains each containing less attractors than the parent domain. The reduction
of localization occurs for at turning point which are critical points of
index 1 located on the separatrix of two basins involved in the parent
domain. Ordering these turning points (localization nodes) by increasing ![]() enables to build tree-diagrams reflecting the hierarchy of the basins.
Three types of domains can be distinguished according to the nature of
the attractors within them. A core domain contains the core attractor(s)
of a given atoms, a valence domain only valence attractors and a composite
domain both valence and core ones. For any system there exist low values
of
enables to build tree-diagrams reflecting the hierarchy of the basins.
Three types of domains can be distinguished according to the nature of
the attractors within them. A core domain contains the core attractor(s)
of a given atoms, a valence domain only valence attractors and a composite
domain both valence and core ones. For any system there exist low values
of ![]() defining a unique composite parent domain. The successive reductions of
localization will split this parent domain. Every child which is a composite
domain corresponds to one or more chemical species. A chemical unit is
the union of the basins of the last appearing composite domain of a branch
provided it is a filled volume.
defining a unique composite parent domain. The successive reductions of
localization will split this parent domain. Every child which is a composite
domain corresponds to one or more chemical species. A chemical unit is
the union of the basins of the last appearing composite domain of a branch
provided it is a filled volume.
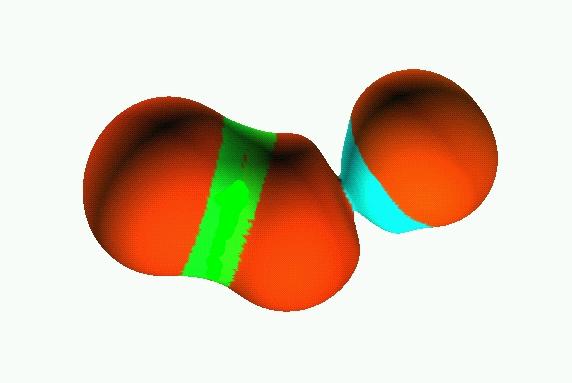
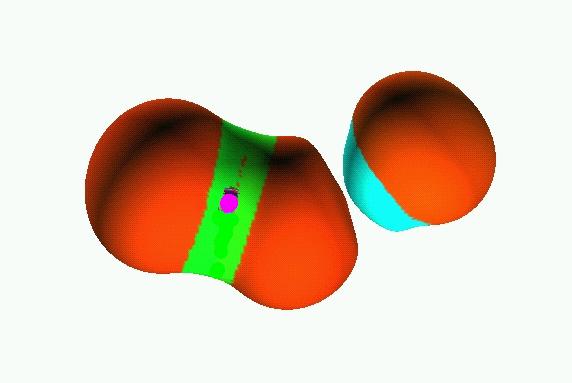
0.05
0.07
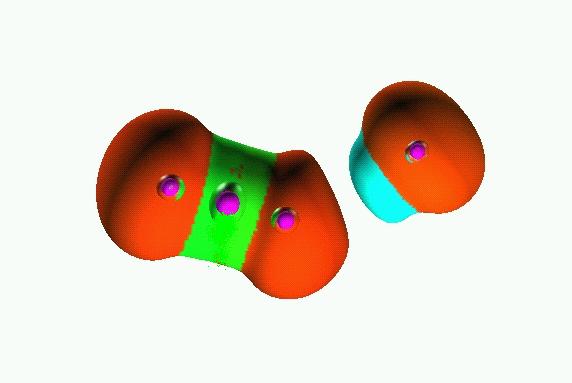
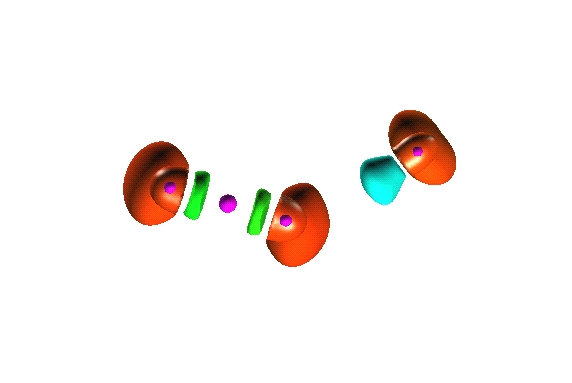
0.25
0.8
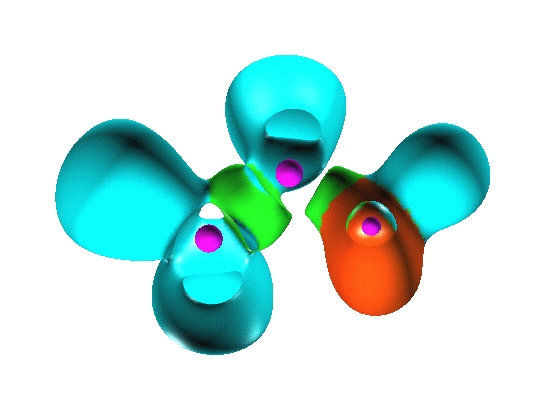
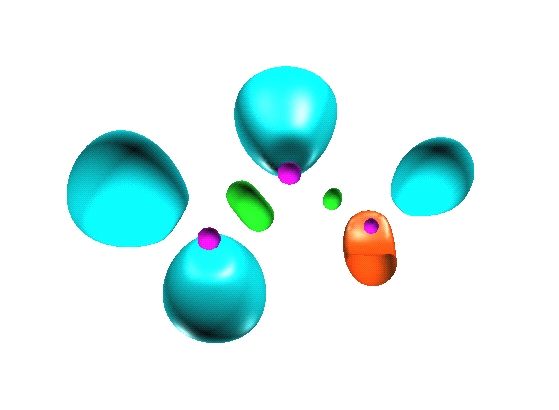
Reduction of localization of the CO2 HF complex.
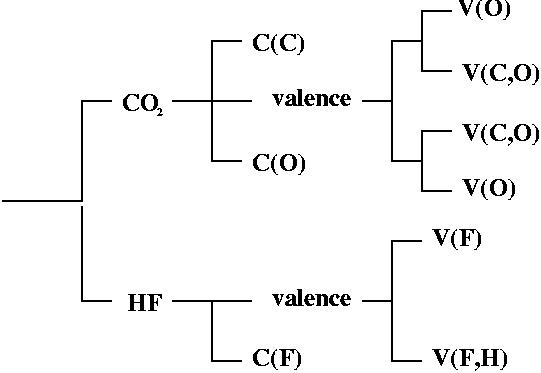
the first reduction
yields two composite domains corresponding to the interacting moieties,
therefore such a complex cannot be considered as being
chemically bonded.
In the same way in an ionic pair the first reduction yields composite domains
corresponding to the cation and to the anion. In a molecule the initial
parent domain first splits into core domains and a single valence domain
which contains all the valence attractors. The shape of this latter domain
is that of a hollowed volume with as many holes as atomic cores in the
molecule. Each hole contain a core domain. The first reductions correspond
to the separation of the core domains from the valence domains which yields
two filled volumes (the core domains) encompassed in a hollowed volume
(the valence domain). In this case there is a unique chemical object.
0.07
0.20
0.55
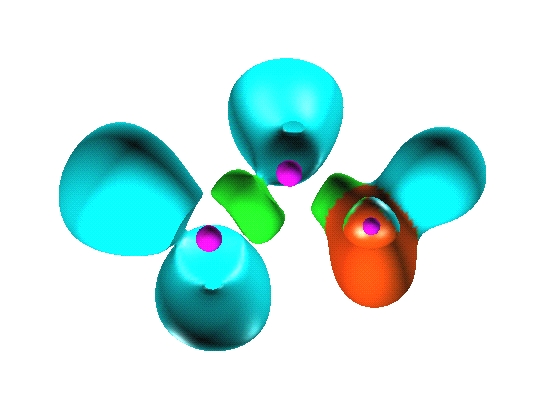
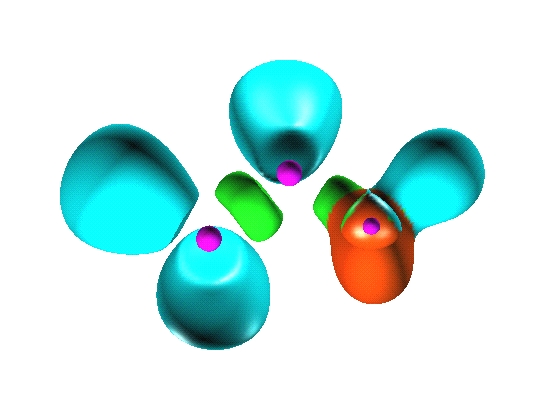
0.66
0.70
0.85
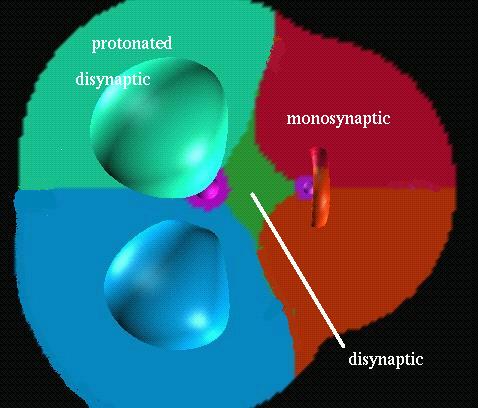
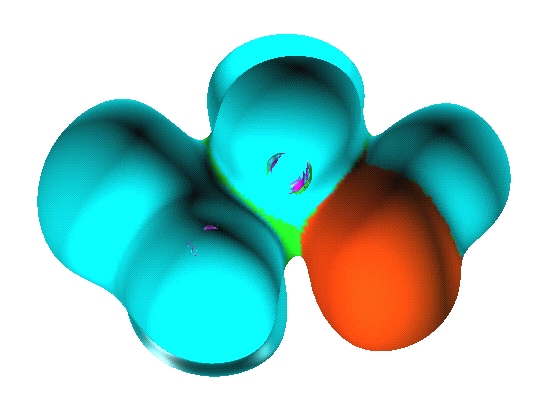
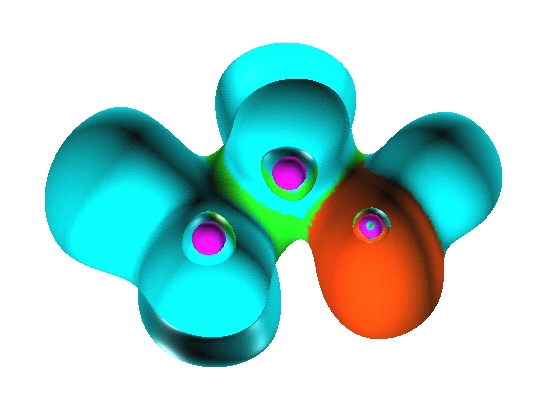
There are seven valence
basins in the CH3F molecules: three protonated
disynaptic V(C,H), one disynaptic V(C,F) and three monosynaptic V(F).
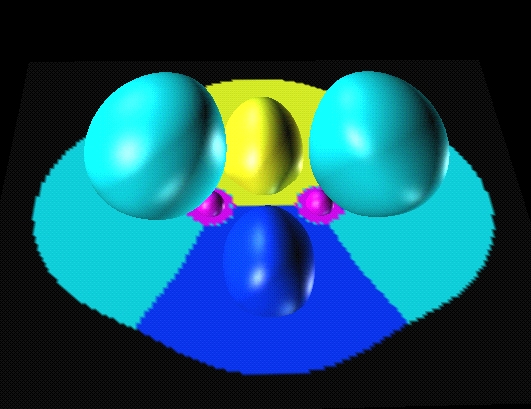
In diborane there are
two protonated trisynaptic basins
(in navy blue and yellow) which correspond to Pitzer's
protonated double bond picture.
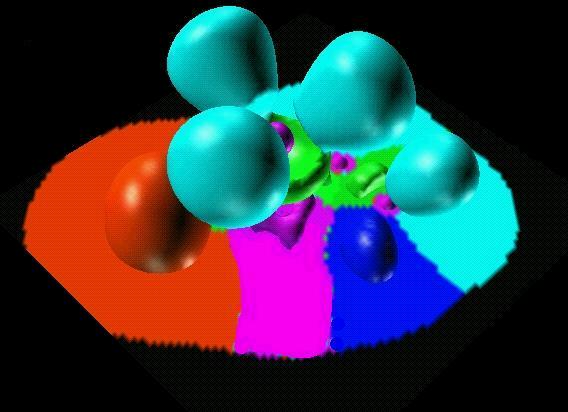
The agostic hydrogen
is also represented by a protonated
trisynaptic basin (in navy blue).
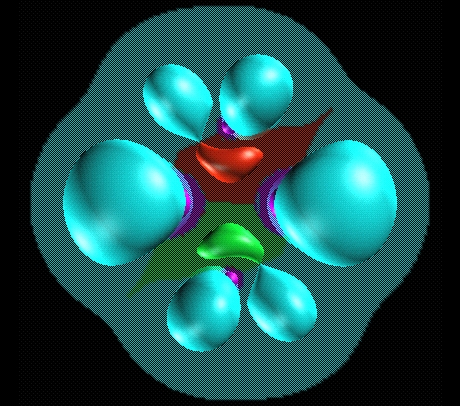
Trisynaptic basins are responsible for anomalous coordination.
Here two pentacoordinated carbon in the Al2H4(CH3)2
molecule.
Superbasins
(cwm)
It often happens that
irreducible domains corresponding to basins of the same type, for example
V(A) monosynaptic basins, are not well separated at the end of a branch.
In this case the value of ![]() at the turning point is very close to the values at the attractors and
it is more chemically meaningful to consider the union of the corresponding
basins rather than the individual basins themselves all the more so that
the number of such basins might be dependent of the quality of the wave
function.
at the turning point is very close to the values at the attractors and
it is more chemically meaningful to consider the union of the corresponding
basins rather than the individual basins themselves all the more so that
the number of such basins might be dependent of the quality of the wave
function.